Abstract
BACKGROUND: Long-term Tirosin Kinase Inhibitor (TKI) treatment is related to notable adverse events, quality-of-life impact and significant costs to health systems. Many patients in optimal response are candidates to TKI discotinuation that has proven to be safe in clinical trials. TFR has become a new goal for CML management. Information about its implementation in clinical practice in LA is limited. The aim of this study is to describe the results, safety and assess the possible economic impact of a cohort of patients that has discontinued TKI in clinical practice in Colombia.
MATERIAL AND METHODS: The Colombian Association of Hematology and Oncology (ACHO)'s hematological disease registry (RENEHOC) is a multicenter nationwide registry on hematologic malignancies that captures information from 18 academic and general community centers with Institutional Ethics Committee approval, since 2018. Since 2019, it has been collecting information on CML. This report represents a sub-analysis of CML patients in the registry in whom discontinuation was performed. Treatment was according to investigator preferences.
A total of 449 CML adult patients treated in the last 20 years have been registered until now on RENEHOC. 29 patients were considered eligible for TFR; in 27 of them, TKI have been discontinued. The main outcome measured is survival without TKI re-initiation.
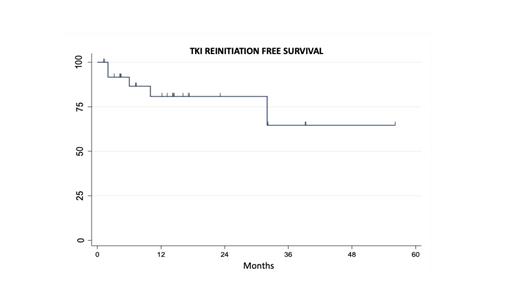
RESULTS: At diagnosis the median age was 58 yrs. (IQR 51-65), all were in the chronic phase and 86% had intermediate-higk risk Sokal score. First line treatment was Imatinib in 11, Dasatinib 9, and Nilotinib 7. 5 patients required a second line with Nilotinib, only one of them was considered to have failed first line. 17 discontinuations were performed as a planned physician strategy, 6 were carried out by patient's decision, 3 were forced by toxicities and in one case it was carried out to search for a pregnancy. Median time on TKIs before discontinuation was 73 months (IQR 59-135) and median time in RMM before TFR was 46 moths (IQR 35-71). Due to its retrospective nature, many patients did not have exact information on MR4.5 achievement date, since it was not available in Colombia until 2016. At a median follow-up of 12.5 months (IQR 4-20), 22 (81%) patients remain on TFR. As has been previously described most patients lost MMR in the first 6 months (median time to restart TKI 6 months; IQR 2-10). Only 1 patient did not achieve the MMR after TKI reinitiated, no progressions to accelerated or blastic phase were reported. 3 patients reinitiated the same ITK and 2 changed to another. 1 patient that was initially treated with Imatinib was changed to Nilotinib after reinitiation and after 2 years in MMR 4.5 has again discontinued TKI and has not lost response after 3 months. 9 (33%) patients developed withdrawal syndrome in most cases (7) with mild symptoms, only 2 had moderate symptoms. The estimated savings on TKIs for Colombian health care system for this patients are US$1.156.937, it is calculated that the cost of the PCR analysis performed for this patients to monitor TFR safely was US$35019.
CONCLUSIONS: TKI has change the landscape for CML patients, that currently have a life expectancy similar to the general population; however, indefinitely treatment is associated with significant toxicities and a very high cost for the systems. TFR has become a real goal for a selected group of patients with CML. This report represents real-world data in Colombia, showing its feasibility and safety under well-controlled settings. Also, the estimated savings for a health care system of a middle-income country as Colombia are very significant, which is an additional support to insist with the decision makers of the system in the importance of the optimal access to TKIs and the necessary tests so that a greater number of patients can reach the necessary goals for a safe TFR.
Abello: Janssen: Honoraria; Amgen: Honoraria; Dr Reddy's: Research Funding. Sossa: Amgen: Research Funding.